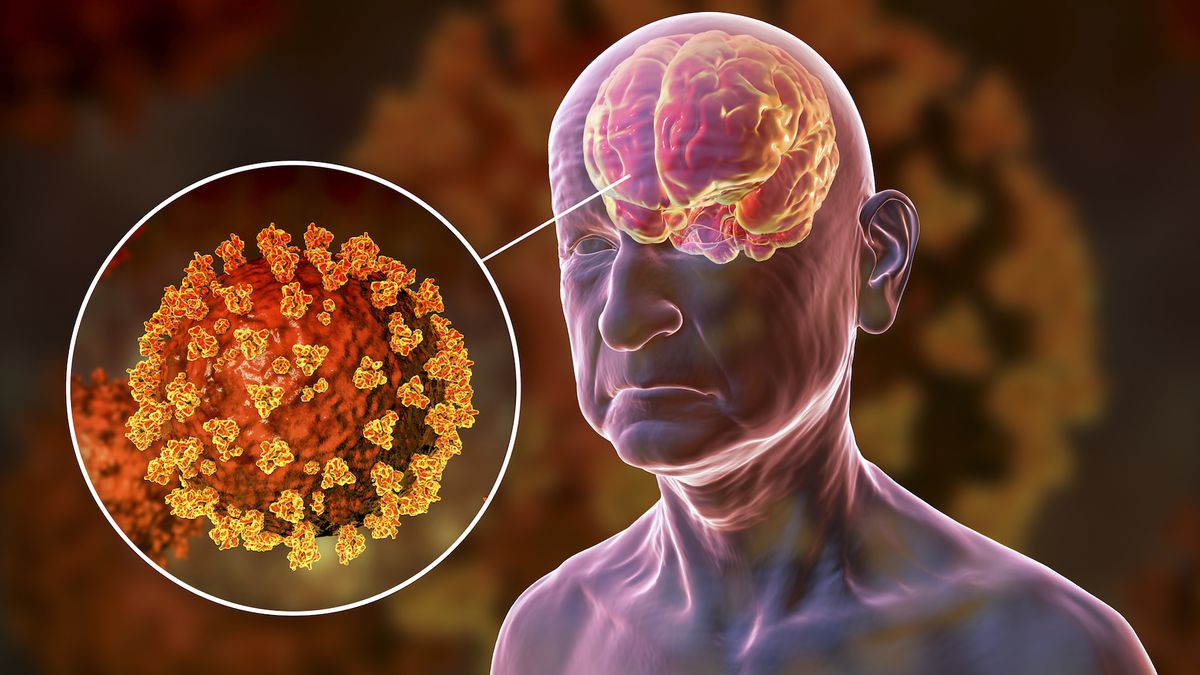
SARS-CoV-2, the virus that causes COVID-19, may preferentially use a “back door” into cells to infect the brain, a new mouse study suggests.
The finding could partly explain why many people have neurological symptoms such as fatigue, dizziness, brain fog, or loss of smell or taste during or after a bout with the virus. Scientists think these symptoms may arise when SARS-CoV-2 enters the central nervous system, but how and why the virus moves from the respiratory tract to the brain wasn’t clear until now.
In an article published Aug. 23 in the journal Nature Microbiology, researchers discovered mutations in the virus’s spike protein, which it uses to enter human cells by binding to a molecule called ACE2 on the cells’ surface.
“The SARS-CoV-2 spike protein coats the outside of the virus and allows it to enter a cell,” study co-author Judd Hultquist, an assistant professor of infectious diseases at Northwestern University in Chicago, told Live Science in an email. “Normally, the virus can enter the cell in two ways: either at the cell surface (through the front door) or internally after it is taken up into the cell (through the back door).”
Part of the spike protein, called the furin cleavage site, helps the virus enter through the front door. If this site is mutated or removed, the virus can only use the backdoor route.
“Cells in the upper airways and lungs are highly susceptible to SARS-CoV-2, which can enter these cells through the front and back doors,” Hultquist said. “To reach and replicate successfully in the brain, it seems like the virus has to enter through the back door. Deleting the furin cleavage site makes the virus more likely to use this pathway — and more likely to infect brain cells.”
To study this, the researchers used genetically engineered mice whose cells make human ACE2. After infecting these mice with SARS-CoV-2, they took virus samples from lung and brain tissue and sequenced the viral genomes.
“We found that mice infected with normal SARS-CoV-2 had some infection in the brain but that there were a lot more infected cells when the virus had a mutation in the furin cleavage site,” Hultquist said. Although it’s not yet possible to say whether these infected cells are responsible for COVID-19’s neurological symptoms, Hultquist and his colleagues saw high rates of infection in cells of the hippocampus and premotor cortex, which are associated with memory and movement, respectively.
However, the study was done only in mice, so more research is needed to find out whether SARS-CoV-2 has similar requirements for infecting the human brain.
“It is important to follow this study up with human sampling to see if the same mutations are found in humans as in mice,” Matthew Frieman, a University of Maryland professor of microbiology and immunology who was not involved in the study, told Live Science. “As researchers target neuronal inflammation for therapy against long-COVID symptoms, understanding how the virus replicates there in the first place is of critical importance.”
Hultquist also wants to know more about why furin cleavage site mutations make the virus more likely to enter the brain. “We show in the study that normal SARS-CoV-2 can replicate in the brain if it is directly injected, which suggests that loss of the furin cleavage site is important for travel to the brain,” Hultquist said. “How exactly this works remains a mystery.”
Even so, this research could lay the groundwork for treating the neurological effects of COVID-19.
“Knowing that the virus needs the back door to infect the brain provides unique opportunities to stop [it],” Hultquist said. “Small molecules that block this pathway may be particularly effective at preventing infection of the brain and the complications that arise. The next challenge will be to figure out not only which drugs may be best able to do this, but also which ones can get to the brain.”
:max_bytes(150000):strip_icc()/John-McCain-Meghan-McCain-Donald-Trump-Kamala-Harris-112124-a94d238beb1f4019a5b06f765a1917fe.jpg)